x-cardiac GmbH has been certified as a medical device manufacturer in the EU while at the same time receiving market authorization for its first medical device x-c-bleeding. The AI-based software can quickly predict the risk of postoperative bleeding in patients. Specialists at the German Heart Center Berlin (DHZB) developed the software and benefited from various support programs of the Berlin Institute of Health at Charité (BIH).
x-cardiac GmbH has been certified as a medical device manufacturer in the EU and is now launching its AI software x-c-bleeding, the first medical device approved for predicting postoperative bleeding. In April x-cardiac GmbH had announced the successful completion of its seed financing round, led by IBB Ventures with the participation of several other business angels.
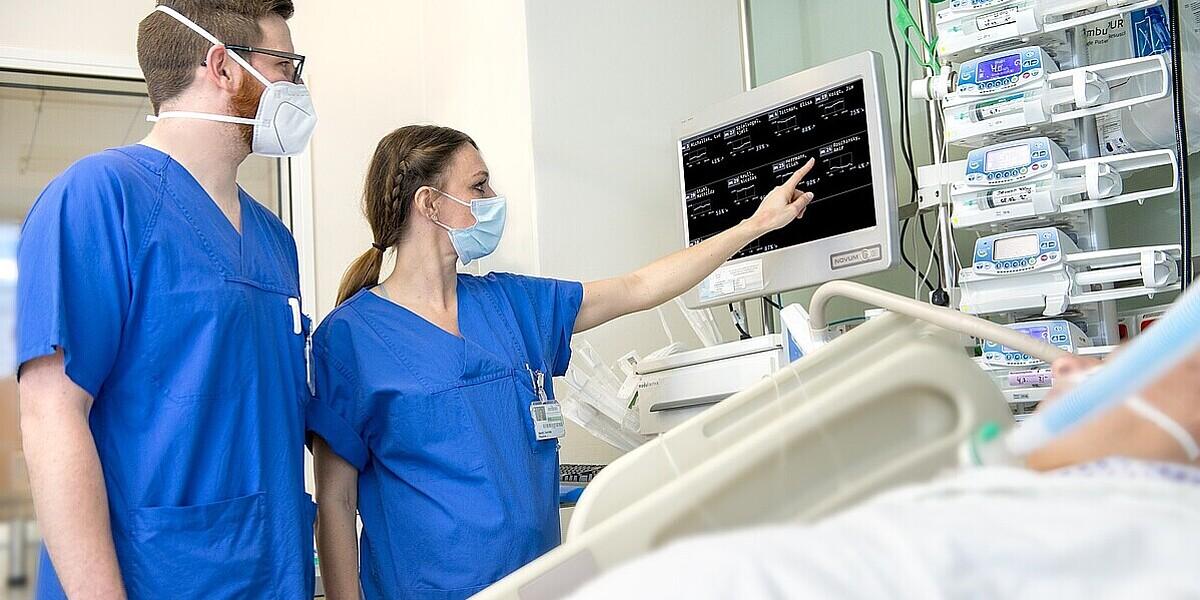
The medical start-up develops AI-based software that helps predict postoperative complications after major heart surgery. The x-cardiac team has “trained” the x-c-bleeding software using anonymized data from nearly 50,000 patients at the DHZB and has since April 2018 been testing the solution in real clinical settings at the DHZB’s intensive care units (ICUs).
“We are thrilled about the approval of our first medical device for commercial use and thank our entire team for the great work they’ve put in to achieve this,” says x-cardiac co-CEO Oliver Höppner. “Our AI-based software enables us to contribute to the much-needed digitalization of daily clinical practice.”
BIH and DHZB supported the venture
Prof. Alexander Meyer, a heart surgeon and computer scientist at the DHZB and co-CEO of x-cardiac, led the development of the software: “There is a need for systems that support clinical decisions at the point of care. In addition, hospitals are facing major challenges when it comes to digitalization. With x-c-bleeding we can provide a market-ready and clinically validated solution. The funding opportunities set out in the German Hospital Future Act (KHZG) make an important contribution here.”
Meyer was supported during the development and testing phase of x-c-bleeding as a fellow of the BIH Charité Clinician Scientist Program. The Validation Fund/Spark-BIH program and the BIH’s Digital Health Accelerator program helped the software take the next steps along the path to deployment and commercialization and, together with the DHZB, supported the founding of x-cardiac GmbH.
Christian Seegers, Senior Investment Manager at IBB Ventures, adds: “With x-c-bleeding, x-cardiac has achieved a well-timed market entry of an excellent medical device that can be used to detect life-threatening postoperative complications earlier than conventional methods. We are delighted that x-cardiac was able to get medical device approval so quickly and can now play a part in digitally transforming the German hospital market.”
The market authorization of its first medical device is an important milestone for x-cardiac as a platform developer for AI-based software in intensive care units. A second medical device called x-c-renal-injury that predicts acute kidney injury (AKI) is already in development. The company is marketing its software with the help of distributors.